
by Dr. Yashashwini Reddy | Oct 9, 2025
In pharmaceuticals, specifications are a set of standards, tests, analytical procedures, and acceptance criteria that define the quality requirements for materials and products. They ensure that every product consistently meets its intended safety, efficacy, and...
by Dr. Yashashwini Reddy | Sep 18, 2025
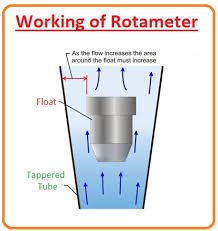
📌 Rotameter A Rotameter is a simple, reliable device used to measure the flow rate of liquids and gases in pharmaceutical, chemical, and other industries. It belongs to the variable area flowmeter family. 🔬 Principle of Rotameter Works on the variable area principle:...
by Dr. Yashashwini Reddy | Sep 16, 2025
1.0 Purpose To define the procedure for the correct operation of the sampling booth to ensure clean, contamination-free, and safe conditions during the sampling of raw materials and to prevent cross-contamination. 2.0 Scope This SOP is applicable for all personnel...

by Dr. Yashashwini Reddy | Sep 15, 2025
📘 Preparation of Annual Product Review (APR) The Annual Product Review (APR) (also referred to as Product Quality Review – PQR in EU) is a regulatory requirement under 21 CFR 211.180(e) and ICH Q7/Q10. Its purpose is to ensure product quality, process consistency, and...

by Dr. Yashashwini Reddy | Sep 15, 2025
Annual Product Quality Review (APQR/APR/PQR) in Quality Improvements 1. What is APQR/APR/PQR? Annual Product Quality Review (APQR), also known as Annual Product Review (APR) or Product Quality Review (PQR), is a regulatory requirement under ICH Q7, EU GMP Chapter 1...







