by Dr. Yashashwini Reddy | Sep 11, 2025
📌 Change Control in Pharmaceuticals Definition:Change Control is a systematic approach to managing all changes made to processes, equipment, systems, or documents in the pharmaceutical industry to ensure compliance with Good Manufacturing Practices (GMP), product...
by Dr. Yashashwini Reddy | Sep 8, 2025
Key Purposes of Change Control in Pharmaceuticals Change control is a critical element of the Quality Management System (QMS) in the pharmaceutical industry. It ensures that all changes affecting processes, equipment, materials, and documents are evaluated,...

by Dr. Yashashwini Reddy | Aug 18, 2025
Quality System in Pharmaceuticals A Quality System in pharmaceuticals is the framework that ensures drugs are consistently developed, manufactured, tested, and distributed in compliance with Good Manufacturing Practices (GMP), regulatory requirements, and patient...

by Dr. Yashashwini Reddy | Aug 12, 2025
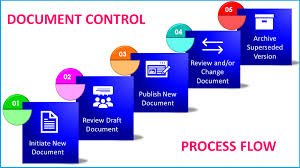
Change Control in Pharmaceuticals Definition:Change control is a formal GMP-compliant system used to manage and document any change in processes, equipment, materials, methods, documents, or facilities to ensure that product quality, safety, and regulatory...
by Dr. Yashashwini Reddy | Aug 9, 2025
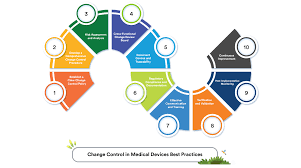
4 Steps to Effective Change Control in Pharmaceuticals 1. Initiation & Documentation Identify the need for change (equipment, process, material, method, etc.). Document the proposed change in a Change Control Form with details like scope, reason, impact area, and...






