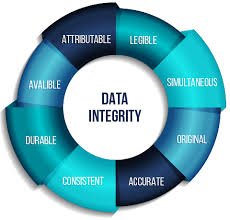
by Dr. Yashashwini Reddy | Sep 15, 2025
Audit Trail Requirements in Pharmaceuticals An audit trail is a secure, computer-generated, time-stamped record that allows for the reconstruction of events related to the creation, modification, or deletion of electronic data. In the pharmaceutical industry, audit...
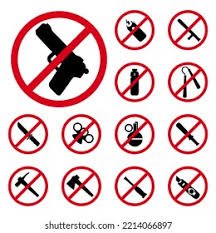
by Dr. Yashashwini Reddy | Apr 27, 2025
Standard Operating Procedure (SOP) 1. Purpose The purpose of this SOP is to provide guidelines for the identification, handling, storage, and disposal of prohibited items within [Organization/Department Name], ensuring compliance with...
by Dr. Yashashwini Reddy | Sep 29, 2024
Top Clinical Data Management Interview Questions and Answers for Freshers 1. What is Clinical Data Management (CDM)? Clinical Data Management is the process of collecting, cleaning, and managing data generated from clinical trials. This ensures the data is accurate,...
by Dr. Yashashwini Reddy | Jul 2, 2024
What is an Audit trail? In this article, we will learn about what an audit trail is, why it is required, and the contents of an audit trail. An audit trail is a 21 CFR Part 11 compliance requirement. These 21 CFR Part 11 deals with electronic records and electronic...





