
by Dr. Yashashwini Reddy | Sep 15, 2025
Self-Inspection and Quality Audits in Pharmaceuticals 1. Self-Inspection Definition: An internal examination carried out by the company itself to evaluate compliance with GMP, SOPs, and regulatory standards. Purpose: Detect deficiencies in the quality system. Verify...

by Dr. Yashashwini Reddy | Sep 9, 2025
🏭 How FDA Inspections are Conducted in Manufacturing Facilities 1. Pre-Inspection Phase FDA identifies facilities to inspect based on: Risk-based selection (product type, compliance history, criticality, recalls, complaints). New drug approval or pre-approval...

by Dr. Yashashwini Reddy | Aug 18, 2025
Top 5 Tips for a Successful FDA Inspection: ✅ Top 5 Tips Be Inspection-Ready Always – Maintain compliance at all times, not just when expecting an inspection. Ensure documentation, training records, SOPs, and facilities are always up-to-date. Ensure Documentation...
by Dr. Yashashwini Reddy | Aug 18, 2025
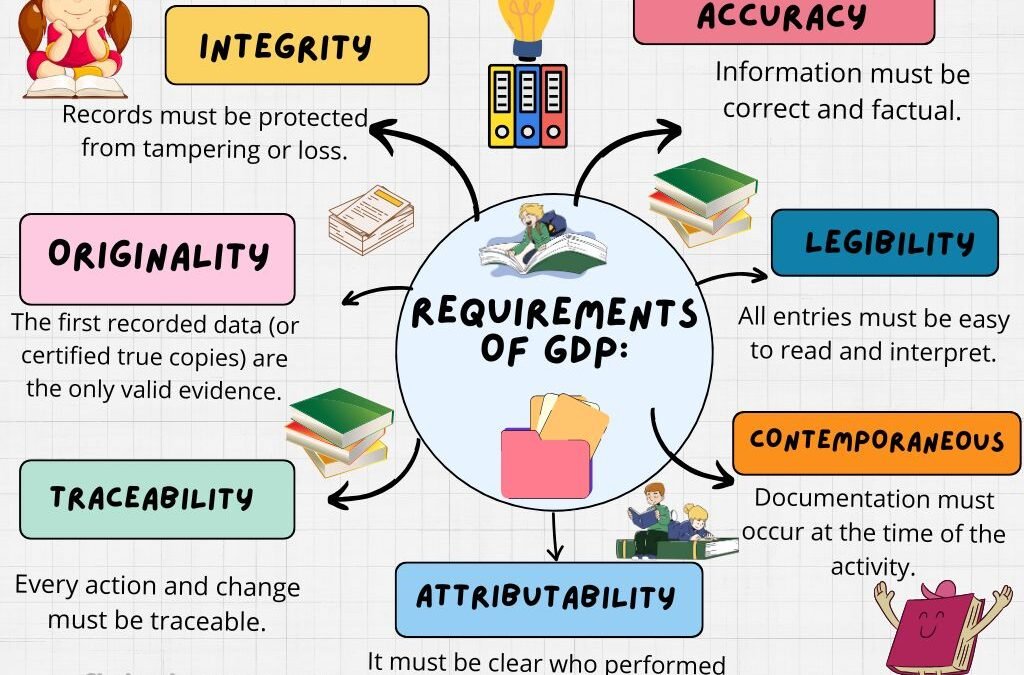
Requirements for Good Documentation Practice (GDP) Legibility All entries should be clear, readable, and permanent (no pencil or erasable ink). Accuracy Data should reflect the actual observation, measurement, or action without manipulation. Contemporaneous Recording...
by Dr. Yashashwini Reddy | Aug 13, 2024
“Understanding Master Copy, Control Copy, and Obsolete Copy in the Pharmaceutical Industry” In the Pharmaceutical industry document issue is very important and every document is traceable and accountable in the form of master copy, control copy, and...






