by Dr. Yashashwini Reddy | Sep 18, 2025
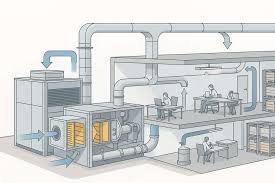
📌 Good Practices for Air Handling Unit (AHU) in Pharmaceuticals 1. Design & Installation AHU must be designed as per GMP & cleanroom classification requirements. Install HEPA/ULPA filters at terminal locations for sterile areas. Provide air locks and pressure...
by Dr. Yashashwini Reddy | Sep 18, 2025
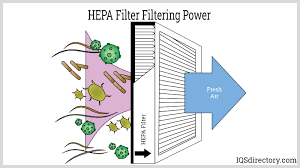
📌 HEPA Filter Uses in Pharmaceutical Manufacturing 🔬 What is a HEPA Filter? High Efficiency Particulate Air (HEPA) Filter removes ≥ 99.97% of airborne particles ≥ 0.3 microns. Essential for cleanroom design and controlled environments. ✅ Uses in Pharmaceutical...
by Dr. Yashashwini Reddy | Sep 2, 2025
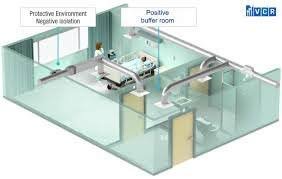
🔹 Buffer Area in Sterile Facility 1. Definition The Buffer Area (also called the cleanroom or critical area) is a Class 100 / ISO 5 / Grade A-B environment where aseptic processing, sterile filling, and direct product exposure operations are performed. It is...
by Dr. Yashashwini Reddy | Sep 2, 2025
📌 Restricted Access Barrier System (RABS) in Pharmaceuticals A Restricted Access Barrier System (RABS) is an advanced containment and protection technology used in pharmaceutical sterile manufacturing to reduce contamination risks. It provides a physical and...

by Dr. Yashashwini Reddy | Aug 27, 2025
Maintenance of Aseptic Conditions in Pharmaceutical Sterile Areas 1. Introduction Sterile areas in pharmaceutical manufacturing (e.g., aseptic filling, compounding, cleanrooms, isolators) require strict control to prevent contamination. Maintaining aseptic conditions...






