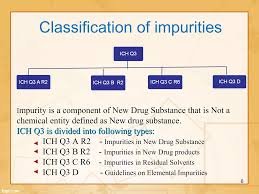
by Dr. Yashashwini Reddy | Oct 8, 2025
Elaboration:Impurities are unwanted chemicals that remain with the active pharmaceutical ingredient (API) or develop during formulation and storage. According to ICH Q3A (for new drug substances) and ICH Q3B (for new drug products), impurities must be identified,...

by Dr. Yashashwini Reddy | Aug 18, 2025
Possible Causes of OOS Results 1. Laboratory Errors Improper sample preparation or dilution Incorrect weighing or pipetting Instrument malfunction or improper calibration Wrong method execution or deviation from SOP Calculation or transcription errors 2....
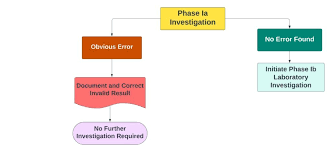
by Dr. Yashashwini Reddy | Aug 12, 2025
Investigation of OOS Results in Analytical Testing 1. Definition An Out of Specification (OOS) result occurs when an analytical test result for a product, raw material, or intermediate falls outside the approved acceptance criteria or specifications set by regulatory...

by Dr. Yashashwini Reddy | Aug 8, 2025
Investigation of OOS Results in Analytical Testing 1. What is an OOS Result? An Out-of-Specification (OOS) result is any analytical test result that falls outside the established acceptance criteria/specifications outlined in an approved method, regulatory filing, or...

by Dr. Yashashwini Reddy | May 19, 2025
A Systematic Investigation of Out-of-Specification (OOS) Results in Analytical Testing is a structured approach to identify, evaluate, and resolve instances where test results fall outside predefined specifications. This process is vital in pharmaceutical quality...







