SOP for Vibro Sifter
Standard Operating Procedure (SOP)
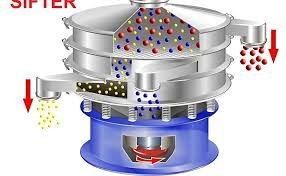
Here is a Standard Operating Procedure (SOP) for a Vibro Sifter, commonly used in pharmaceutical, food, and chemical industries for particle separation and sizing.
1. Purpose
To describe the standard method for operating and cleaning the Vibro Sifter used for separating or sieving materials based on particle size.
2. Scope
This SOP applies to all production personnel responsible for using the Vibro Sifter.
3. Responsibility
-
Machine Operator: Operate and clean the Vibro Sifter as per procedure.
-
Production Supervisor: Ensure compliance and proper training.
-
QA/QC Department: Verify cleanliness and proper documentation.
4. Equipment/Materials Required
-
Vibro Sifter
-
Sieve of required mesh size
-
Collection container
-
PPE (gloves, mask, hairnet, apron, goggles)
-
Cleaning materials and tools
5. Procedure
5.1 Pre-Operation Checks
-
Ensure the equipment is clean, dry, and labeled as “Cleaned.”
-
Check for the correct mesh screen installation and proper assembly.
-
Inspect gaskets, clamps, and lid tightness.
-
Verify the area is clean and the power supply is off.
5.2 Operation Procedure
-
Wear appropriate PPE.
-
Switch ON the power supply and then start the machine.
-
Feed material uniformly into the sifter hopper.
-
Monitor the sifting process—ensure no clogging or vibration issues.
-
Collect sifted material in clean, labeled containers.
-
Stop feeding once material is fully processed.
-
Turn OFF the machine and then the power supply.
5.3 Post-Operation
-
Remove any remaining material.
-
Attach “To Be Cleaned” status label if not cleaned immediately.
-
Log operational details in the equipment logbook.
5.4 Cleaning Procedure
-
Disconnect power before cleaning.
-
Dismantle contact parts: cover, sieve, and container ring.
-
Wash all dismantled parts with purified water and approved detergent.
-
Rinse thoroughly and allow to dry in a controlled environment.
-
Wipe the non-contact parts with a clean, damp cloth.
-
Reassemble or store cleaned parts properly.
-
Record cleaning activity in the logbook.
6. Safety Precautions
-
Do not operate without securing the lid and clamps.
-
Never insert hands during operation.
-
Always wear PPE and handle meshes with care.
-
Report unusual vibration, noise, or performance issues.
7. Documentation
-
Equipment usage log
-
Cleaning log
-
Batch Manufacturing Record (BMR)
-
Cleaning checklist
🎓 Discover one of the best Pharmaceutical Production courses available — click below to explore the course that’s shaping future Production skills.

