SOP for Preparation, Sterilization, Storage and Growth Promotion Testing of Solid and Liquid Media
Title: SOP for Preparation, Sterilization, Storage, and Growth Promotion Testing of Solid and Liquid Media
1.0 Objective
To provide a standardized method for preparation, sterilization, storage, and growth promotion testing of microbiological media used for microbial testing.
2.0 Scope
This SOP applies to the preparation of both solid and liquid media required for microbiological testing procedures.
3.0 Responsibility
Microbiologist: Following procedures in this SOP, documenting work, and reporting deviations.
QCD in charge/Supervisor: Overseeing implementation of this SOP, providing resources and training.
Quality Assurance: Approving the SOP, evaluating investigations and efficacy of corrective actions.
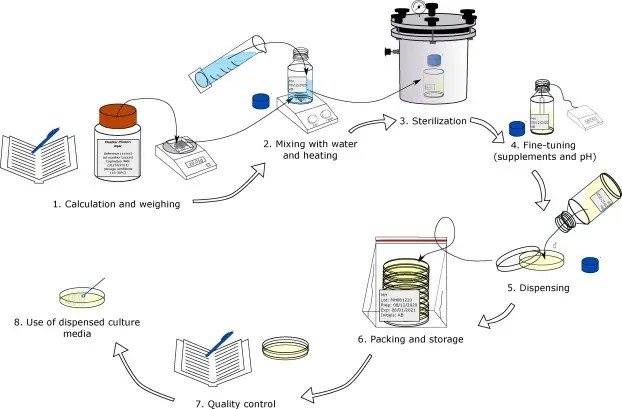
4.0 Procedure
4.1 General Instructions
4.1.1 Use clean, inspected glassware and accessories for all media preparation activities.
4.1.2 Ensure weighing balances, pH meters, autoclaves are properly calibrated before use.
4.1.3 Use only purified water or WFI (Water for Injection) for reconstituting the dehydrated media.
4.1.4 Carefully inspect dehydrated media containers for instructions, use-by date, absence of clumps/caking before use.
4.1.5 Do not keep dehydrated hygroscopic media open for extended periods.
4.1.6 Prepare media exactly as per manufacturer’s instructions, do not overheat or re-melt solidified media more than 1 time.
4.1.7 Store all dehydrated and prepared media at recommended conditions.
4.1.8 Wear proper PPE like gloves, masks when handling media, especially selective/toxic media.
4.2 Procurement and Inhouse Testing of Dehydrated/Ready-to-Use Media
4.2.1 Procure required quantity of dehydrated or ready-to-use culture media from approved, reputed vendors like HI Media.
4.2.2 Enter all incoming media details in the dehydrated media consumption log.
4.2.3 Upon receipt, verify name, code, lot/batch number, storage conditions, instructions, use-by date, Certificate of Analysis against each container.
4.2.4 Perform inhouse quality tests upon receipt of dehydrated media:
– Visual appearance
– Solubility
– Appearance of reconstituted medium
– pH before and after sterilization/autoclaving
– Growth promotion testing (including inhibition for selective media).
4.2.5 If all tests meet CoA specifications, accept media and label containers with:
– Receipt date
– Container number
– Analyst initials
Store accepted containers at recommended conditions.
4.2.6 For opened containers, label with opening date and initials. Discard if appearance is unsatisfactory.
4.3 Liquid Media Preparation
4.3.1 Use dedicated glassware for each liquid media type.
4.3.2 Check batch number, use-by date and record details in media preparation log.
4.3.3 Calculate total quantity of each ingredient required based on desired final volume.
4.3.4 Weigh out the required quantity of dehydrated media powder for the desired volume as per instructions on the bottle provided by the manufacturer.
4.3.5 Dissolve powder by heating with continuous stirring. For large volumes, add remaining water to make up final volume and mix well.
4.3.6 Adjust pH if needed using 0.1N HCl or 0.1N NaOH.
4.3.4 Assign lot number to prepared media as per lot numbering.
4.3.7 After sterilization, check pH from one container and discard it. pH should be within specified limits.
4.3.8 Distribute media into appropriate glass bottles for use.
4.3.9 Plug containers with cotton plugs or metal caps or Autoclavable plastic caps.
4.3.10 Affix label with media details on each container.
4.3.11 If using cotton plugs, cover with wrapping paper (butter paper or parchment paper).
4.3.12 Unless specified otherwise, sterilize at 121°C for 20 minutes as per sterilization SOP.
4.3.13 After sterilization, allow media to cool to room temperature before proceeding to pre-incubation and growth promotion testing.
4.4 Solid Media Preparation
4.4.1 Weigh out specified quantity of dehydrated media powder into a beaker with double the final volume capacity.
4.4.2 Add appropriate quantity of distilled water and mix well.
4.4.3 Assign lot number as per lot numbering SOP.
4.4.4 Heat with constant stirring to dissolve media components fully and avoid charring.
4.4.5 Check and adjust pH using 0.1N HCl or 0.1N NaOH if required.
4.4.6 While molten, distribute specific volumes into appropriate glass containers.
4.4.7 Seal container mouths with cotton plugs or metal caps or Autoclavable plastic caps.
4.4.8 If using cotton plugs, cover with wrapping paper (butter paper or parchment paper).
4.4.9 Unless specified, sterilize at 121°C for 20 minutes as per sterilization SOP. After sterilization, allow molten agar to cool to 45-50°C.
4.4.10 After sterilization, use solid media in desired form:
- A) For slants – Allow to solidify in slanted position
- B) For stabs – Allow to solidify in upright position
- C) For plates – Pour into sterile petri dishes in laminar air flow and allow to solidify
4.4.11 To maintain molten state, keep media at 45-50°C in oven or water bath until use (NMT 8 hrs or as per validated period).
4.4.12 Store sterile plates/ slants/stabs at 20-25°C up to 7 days or as per validated period.
4.5 Pre-incubation
4.5.1 Liquid Media
– Pre-incubate all prepared media from each lot at 30-35°C for 48 hrs.
– Fungal media shall be pre incubated at 20-25°C for 48 hrs.
– Acceptance criteria: No turbidity/contamination
– Discard and investigate entire lot if contamination is observed more than 10.
4.5.2 Agar Media
– Pre-incubate 100% of plates at 30-35°C for 48 hrs.
– Fungal media shall be pre incubated at 20-25°C for 48 hrs.
– Check plates for defects like breakage, improper volume, particulates, Air bubbles, contamination, improper convexity (55mm plates)
– Discard defective plates
– Acceptance: No more than 5% contaminated plates per lot. Investigate if higher rate.
4.6 Sterility Testing
4.6.1 Agar Media
– After pre-incubation, incubate 2 plates (<100 plates) or 5 plates (>100 plates) from each lot at test conditions specified for that media type.
4.6.2 Liquid Media
– Incubate pre-incubated containers at specified test conditions.
4.6.3 Acceptance criteria: No growth/contamination
4.6.4 Investigate entire lot if growth/contamination is observed
4.6.5 Record details in media preparation log
4.7 Growth Promotion Testing
4.7 Growth Promotion and Inhibition (Selectivity) Testing
4.7.1 Perform growth promotion testing on each lot of prepared/ready-to-use media
4.7.2 For selective media, also perform inhibition testing
4.7.3 Use standardized inoculum prepared as per inoculum SOP
4.7.4 Test specified control organisms, incubation conditions, and evaluation criteria as per media type
4.7.5 Solid Media
– Inoculate 10-100 CFU in duplicate by pour/spread plate method
– General Media: 50-200% recovery of inoculum count within specified duration
– Selective/Differential Media: Characteristic growth within specified duration
4.7.6 Liquid Media
– Inoculate 10-100 CFU in duplicate
– Observe for visible turbidity after incubation
– Selective/Differential: Characteristic growth
4.7.7 After growth promotion testing, transfer all pre-incubated sterile liquid and solid media to 20-25°C for use.
4.7.8 Inhibition Test (Selective/Differential Media)
– Inoculate at least 100 CFU of specified inhibition organism in duplicate
– Incubate at specified conditions for not less than the duration specified
– Acceptance criteria: No growth of the inhibition organism(s)
4.8 Labeling and Storage
4.8.1 Assign unique lot numbers to each prepared media lot as per following format:
ABCXXX
Where ABC = Unique 3 letter media code
XXX = Sequential lot number starting 001 for each calendar year
E.g. First Mannitol Salt Agar lot of the year = MSA001
4.8.2 Label packs/stacks of plates, tubes, bottles with:
– Media name
– Lot number
– Preparation date
– Use-by/Expiry date
– Analyst initials
4.8.3 Store prepared media at recommended conditions:
– Sterile plates at 20-25°C
– Sterile liquid media at 20-25°C
– Sterile slants/stabs at freezer temperature
4.9 Shelf Life and Use
4.9.1 Do not use media more than 3 weeks old for sterility testing purposes.
4.9.2 Use other media within 1 month when stored at 20-25°C.
4.9.3 Use slants and stabs within 2 months when stored at freezer temperature.
4.7.1 Do not use media more than 1 weeks old for sterility testing.
4.7.2 Use media other than for sterility testing within 15 days when stored at 20-25°C.
4.7.3 Maintain media preparation records for traceability of use.
5.0 Abbreviations
5.1 SOP – Standard Operating Procedure
5.2 PPE – Personal Protective Equipment
5.3 WFI – Water for Injection
5.4 CoA – Certificate of Analysis
5.5 CFU – Colony Forming Units
5.6 °C – Degree Celsius
5.7 ml – Milliliter
5.8 NMT = Not More Than
6.0 References
6.1 Applicable Pharmacopoeia monographs (USP/EP/WHO)
6.2 Media manufacturer’s instructions
6.3 SOP for pH measurement
6.4 SOP for sterilization/autoclaving
6.5 SOP for inoculum preparation
6.6 SOP for biosafety cabinet/laminar operations
7.0 Annexures
7.1 Media Preparation Log
7.2 Growth Promotion Testing Log
7.3 Dehydrated media consumption log
- Author: Bhavana Tatineni
- Qualification: MSc Microbiology

