SOP for Microbiological Monitoring of Clean
Standard Operating Procedure (SOP) for Microbiological Monitoring of Cleanroom
1. Purpose
To describe the procedure for microbiological monitoring of cleanrooms to ensure controlled aseptic conditions and compliance with GMP guidelines.
2. Scope
This SOP applies to all classified cleanrooms (Grade A, B, C, and D) in the facility used for sterile manufacturing, processing, and support operations.
3. Responsibility
-
Microbiologist/Analyst: Perform microbiological monitoring, record observations, report deviations.
-
QC Supervisor: Review and verify results, initiate investigation in case of excursions.
-
QA: Ensure compliance, review trend reports, approve results.
-
Production: Facilitate monitoring during operations.
4. Materials & Equipment
-
Settle plates (TSA, SDA, or as validated)
-
Contact plates (RODAC plates)
-
Active air sampler
-
Sterile swabs
-
Incubators (20–25°C & 30–35°C)
-
Sterile gowns, gloves, PPE
-
EM logbook / electronic data system
5. Procedure
5.1 Sampling Plan
-
Grade A & B: Daily during each batch operation.
-
Grade C & D: As per frequency defined by risk assessment (weekly/monthly).
-
Locations include: working areas (LAFs, isolators, filling line), background clean areas, gowning rooms, and airlocks.
5.2 Methods of Monitoring
-
Settle Plates – Expose for up to 4 hours in critical and non-critical locations.
-
Active Air Sampling – Collect defined volume of air (e.g., 1 m³) using calibrated air sampler.
-
Contact Plates (Surface Monitoring) – Press RODAC plates onto cleanroom surfaces (walls, floors, equipment).
-
Swab Sampling – For irregular/non-flat surfaces.
-
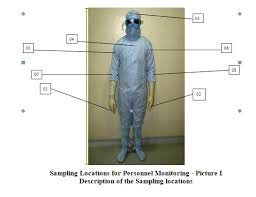
Personnel Monitoring – Finger dab plates and gown surface monitoring post-operation.
5.3 Incubation Conditions
-
Bacterial media (TSA/SCDA): 30–35°C for 48–72 hrs.
-
Fungal media (SDA): 20–25°C for 5–7 days.
5.4 Acceptance Criteria (as per EU GMP Annex 1)
| Grade | Air (cfu/m³) | Settle plate 90mm (cfu/4hr) | Contact plate (cfu/plate) | Glove print (5 fingers, cfu/glove) |
|---|---|---|---|---|
| A | <1 | <1 | <1 | <1 |
| B | 10 | 5 | 5 | 5 |
| C | 100 | 50 | 25 | NMT 25 |
| D | 200 | 100 | 50 | NMT 50 |
5.5 Post-Monitoring Activities
-
Record results in EM logbook or system.
-
Identify isolates if results exceed alert/action limits.
-
Initiate deviation/investigation for excursions.
-
Compile data in monthly/quarterly trend reports.
6. Precautions
-
Perform monitoring aseptically to avoid introducing contamination.
-
Use only sterile, validated media.
-
Avoid obstructing unidirectional airflow while sampling.
-
Handle all plates carefully to avoid accidental contamination.
7. Documentation
-
Record location, date, media lot, exposure time, incubation details, and results.
-
Maintain trending charts for EM program.
-
Attach investigation reports for deviations.
8. References
-
EU GMP Annex 1 (2022)
-
USP <1116> Microbiological Control and Monitoring of Aseptic Processing Environments
-
ISO 14698 (Biocontamination Control)
-
WHO TRS Guidelines
🎓 Discover one of the best Pharmaceutical Microbiology course available —click below to explore the course that’s shaping future Microbiology course skills.
#GMPCompliance, #LabEquipment, #PharmaQuality, #PharmaSOP, #StandardOperatingProcedure
Posted onAugust 30, 2025

