Self Inspection and Its Implementation in Pharmaceuticals
Self-Inspection and Its Implementation in Pharmaceuticals
Definition:
Self-inspection is a systematic, independent, and documented internal audit conducted by a pharmaceutical company to evaluate compliance with Good Manufacturing Practices (GMP), regulatory requirements, and internal procedures.
Objectives of Self-Inspection
-
To ensure compliance with GMP and SOPs.
-
To identify weaknesses, deviations, or non-conformities.
-
To promote continuous improvement in processes and systems.
-
To prepare the organization for regulatory audits (FDA, EMA, WHO, etc.).
-
To build a strong quality culture.
Scope of Self-Inspection
Covers all areas directly or indirectly affecting product quality, including:
-
Production areas
-
Quality Control (QC) laboratories
-
Warehousing and material management
-
Documentation and records
-
Utilities (HVAC, Water System, Compressed Air)
-
Validation and calibration activities
-
Training and personnel hygiene
-
Distribution practices
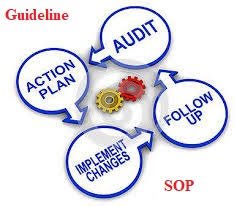
Implementation Steps
-
Planning and Scheduling
-
Define the frequency (monthly, quarterly, yearly).
-
Prepare a self-inspection schedule.
-
-
Formation of Self-Inspection Team
-
Independent and trained personnel from QA, QC, Production, Engineering.
-
Sometimes external experts may be included.
-
-
Execution of Inspection
-
Follow a checklist covering GMP areas.
-
Collect observations, review practices, and interview staff.
-
-
Documentation of Findings
-
Record all observations (critical, major, minor).
-
Use standard reporting templates.
-
-
Corrective and Preventive Actions (CAPA)
-
Assign responsibilities and timelines.
-
Ensure implementation and effectiveness checks.
-
-
Review and Follow-Up
-
QA to verify closure of CAPA.
-
Report to senior management for continuous improvement.
-
Benefits of Self-Inspection
-
Ensures readiness for regulatory audits.
-
Identifies risks before they escalate.
-
Strengthens compliance and data integrity.
-
Improves efficiency and accountability.
-
Enhances overall product quality and patient safety.
🎓 Discover one of the best Pharmaceutical Quality Assurance course available —click below to explore the course that’s shaping future Quality Assurance skills.
https://trcjw.on-app.in/app/oc/306166/trcjw

