Requirements for Good Documentation Practice (GDP)
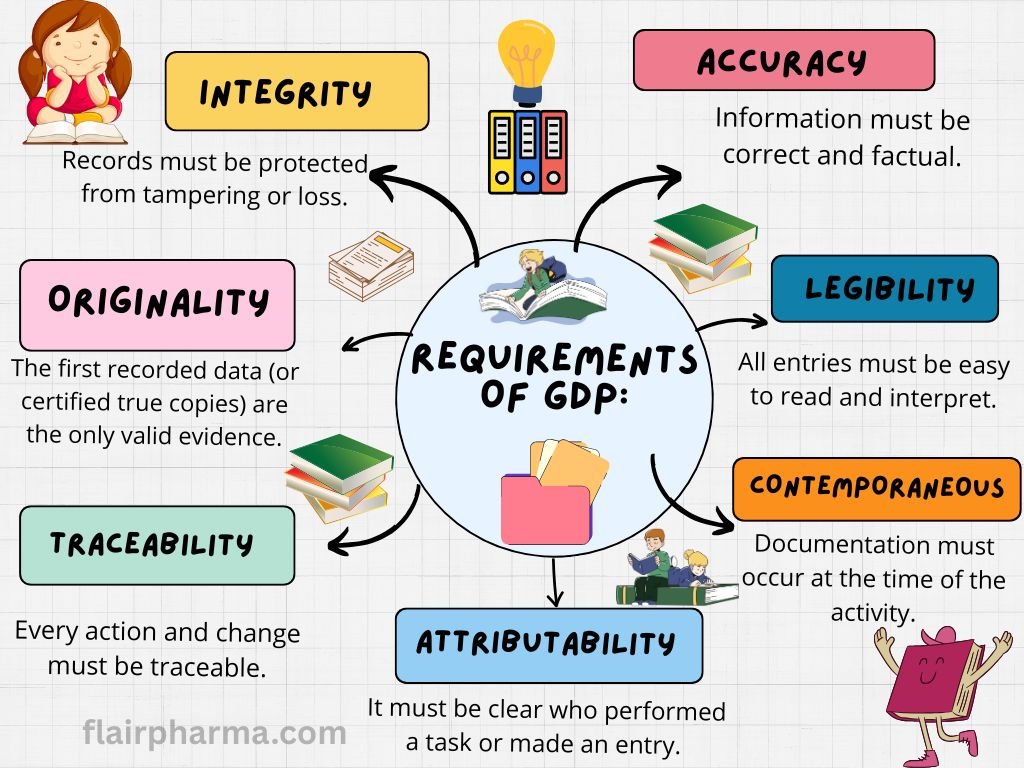
Requirements for Good Documentation Practice (GDP)
-
Legibility
-
All entries should be clear, readable, and permanent (no pencil or erasable ink).
-
-
Accuracy
-
Data should reflect the actual observation, measurement, or action without manipulation.
-
-
Contemporaneous Recording
-
Entries should be made at the time the activity is performed, not afterward.
-
-
Attributable
-
Every entry must be traceable to the person making it, usually through signatures, initials, or electronic login.
-
-
Original
-
Data should be recorded directly into controlled documents or authorized formats (not on scrap paper).
-
-
Complete
-
No information should be omitted; corrections must not obscure the original entry.
-
-
Consistent
-
Dates, formats, terminologies, and units of measurement should be used consistently.
-
-
Corrections
-
Errors must be corrected with a single line through the incorrect entry, signed/initialed, dated, and explained if needed (no overwriting).
-
-
Controlled Documents
-
Use only approved, version-controlled documents; obsolete versions should be withdrawn.
-
-
Audit Trail
-
For electronic records, there must be a secure and traceable audit trail to capture changes.
-
-
ALCOA+ Principles
-
Data must be Attributable, Legible, Contemporaneous, Original, Accurate, and also Complete, Consistent, Enduring, Available.
-
🎓 Discover one of the best Pharmaceutical Quality Assurance course available —click below to explore the course that’s shaping future Quality Assurance skills.
https://trcjw.on-app.in/app/oc/306166/trcjw

