Quality by Design (QbD) in Pharmaceuticals
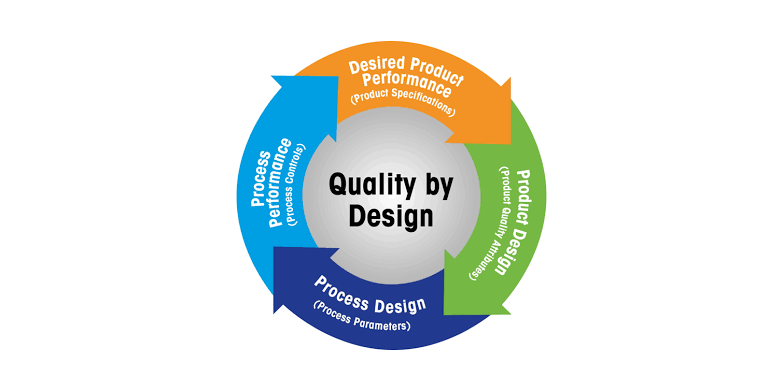
Quality by Design (QbD) in Pharmaceuticals
Definition:
Quality by Design (QbD) is a systematic, risk-based, proactive approach to pharmaceutical development that emphasizes designing and building quality into the product from the very beginning, rather than relying solely on final product testing.
Key Principles of QbD
-
Define Quality Target Product Profile (QTPP)
-
Identify the intended use, dosage form, route of administration, strength, and patient safety requirements.
-
-
Identify Critical Quality Attributes (CQAs)
-
Physical, chemical, biological, or microbiological characteristics that must be within limits to ensure product quality.
-
-
Determine Critical Material Attributes (CMAs) & Critical Process Parameters (CPPs)
-
Material and process variables that affect CQAs.
-
-
Risk Assessment
-
Use tools like FMEA (Failure Mode and Effects Analysis), Ishikawa diagrams, and risk ranking to identify and prioritize risks.
-
-
Design Space
-
The multidimensional range of CMAs and CPPs that assure quality — approved by regulators as part of the control strategy.
-
-
Control Strategy
-
Planned set of controls (material specifications, process parameters, in-process controls, and end-product testing).
-
-
Continuous Improvement
-
Ongoing monitoring and process optimization throughout the product lifecycle.
-
Benefits of QbD
-
Reduces development time and cost.
-
Improves product consistency and patient safety.
-
Minimizes regulatory hurdles for post-approval changes.
-
Enhances understanding of product and process.
Regulatory Perspective
-
Endorsed by ICH guidelines:
-
ICH Q8: Pharmaceutical Development
-
ICH Q9: Quality Risk Management
-
ICH Q10: Pharmaceutical Quality System
-

