Q9: Quality Risk Management
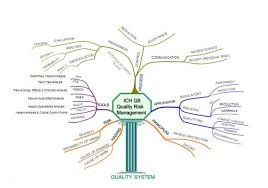
Quality Risk Management (QRM) is a systematic process for the assessment, control, communication, and review of risks to the quality of a pharmaceutical product across its lifecycle. The purpose of QRM is to ensure that product quality, patient safety, and regulatory compliance are maintained through proactive identification and mitigation of potential risks.
Key Principles (as per ICH Q9):
-
Risk Assessment – Identifying hazards, analyzing and evaluating risks associated with exposure to those hazards.
-
Tools: FMEA (Failure Mode and Effects Analysis), Fault Tree Analysis (FTA), Hazard Analysis and Critical Control Points (HACCP), Risk Ranking, and HAZOP.
-
-
Risk Control – Deciding which risks need reduction and implementing appropriate control measures.
-
Risk Communication – Sharing information among stakeholders regarding risk and its management.
-
Risk Review – Continuous monitoring and review of risks throughout the product lifecycle.
Application Areas:
-
Change control
-
Deviation and CAPA management
-
Supplier qualification
-
Equipment qualification
-
Validation (Process/Analytical/Computer system)
-
Investigation and batch disposition decisions
Benefits of QRM:
-
Enhances product quality and patient safety
-
Promotes scientific and data-driven decision-making
-
Improves resource utilization and efficiency
-
Supports continual improvement in the Quality Management System (QMS)

