Q1: Stability Testing
📘 ICH Q1: Stability Testing
🔹 Purpose
The ICH Q1 series provides guidance on how to design stability studies to ensure that drug substances and drug products maintain their quality, safety, and efficacy throughout their shelf life under various environmental conditions.
Stability testing helps determine:
-
Re-test period for drug substances.
-
Shelf life / expiry date for drug products.
-
Recommended storage conditions (e.g., “Store below 25°C”).
🧪 Key ICH Q1 Guidelines and Their Focus
| Guideline | Title / Focus | Purpose |
|---|---|---|
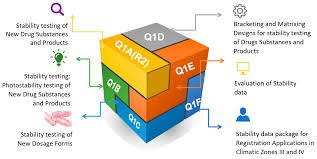
| Q1A(R2) | Stability Testing of New Drug Substances and Products | General framework for designing stability studies, testing frequency, and storage conditions. |
| Q1B | Photostability Testing of New Drug Substances and Products | Evaluates the effect of light exposure on drug stability. |
| Q1C | Stability Testing for New Dosage Forms | Describes requirements for stability testing when new dosage forms are developed. |
| Q1D | Bracketing and Matrixing Designs for Stability Testing | Provides statistical approaches to reduce the number of stability tests without compromising data integrity. |
| Q1E | Evaluation for Stability Data | Guidance for the statistical evaluation of stability data to determine shelf life. |
| Q1F | Stability Data Package for Registration Applications in Climatic Zones III and IV | Specifies conditions for hot and humid regions (e.g., 30°C/65% RH and 30°C/75% RH). |
🌡️ Typical Stability Storage Conditions
| Condition | Temperature & Humidity | Duration |
|---|---|---|
| Long-term | 25°C ± 2°C / 60% RH ± 5% | 12 months (min) |
| Intermediate | 30°C ± 2°C / 65% RH ± 5% | 6 months |
| Accelerated | 40°C ± 2°C / 75% RH ± 5% | 6 months |
Special cases:
-
Refrigerated products: 5°C ± 3°C
-
Freezer products: -20°C ± 5°C
-
Photostability: Exposed to defined light intensity (UV + visible)
📈 Importance of Stability Testing
-
Ensures consistent quality throughout product lifecycle.
-
Determines appropriate packaging and storage conditions.
-
Helps establish expiry date and retest period.
-
Ensures compliance with regulatory submissions (CTD Module 3).
-
Detects degradation products and ensures they remain within acceptable limits.
⚙️ Types of Stability Studies
-
Real-Time Stability Studies – Conducted under recommended storage conditions for the full shelf life.
-
Accelerated Stability Studies – Conducted at elevated temperature and humidity to predict shelf life.
-
Intermediate Studies – Conducted when results from accelerated studies show significant change.
-
Photostability Studies – Evaluate the effect of light exposure.
🧩 Key Points to Remember
-
Use validated and stability-indicating analytical methods.
-
Follow Good Storage Practices (GSP) for samples.
-
Maintain control samples for comparison.
-
Document all results in a Stability Summary Report.
-
Any significant change requires investigation and justification.

