PH Value, pH Scale and Its Measurement between 0 and 14
pH Value, pH Scale, and Its Measurement (0–14)
1. pH Value
-
Definition: pH is a measure of the hydrogen ion concentration [H+][H⁺] in a solution, indicating its acidity or alkalinity.
-
Formula:

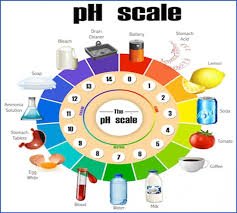
2. pH Scale (0–14)
| pH Range | Nature | Example |
|---|---|---|
| 0–3 | Strongly acidic | Battery acid, gastric acid |
| 4–6 | Weakly acidic | Tomato juice, black coffee |
| 7 | Neutral | Pure water |
| 8–10 | Weakly alkaline | Baking soda solution |
| 11–14 | Strongly alkaline | Bleach, soapy water |
3. Measurement of pH
A. pH Meter Method (Most Accurate)
-
Steps:
-
Calibrate the pH meter with standard buffer solutions (usually pH 4.0, 7.0, and 10.0).
-
Rinse electrode with distilled water.
-
Immerse the electrode in the sample.
-
Record the reading when stabilized.
-
-
Advantages: High accuracy, quick measurement.
B. Indicator Paper (pH Paper)
-
Color change compared with a standard chart.
-
Suitable for quick, approximate readings.
C. Chemical Indicators
-
Use pH-sensitive dyes (e.g., phenolphthalein, methyl orange).
-
Mostly for titrations and qualitative analysis.
4. Factors Affecting pH Measurement
-
Temperature of solution (pH varies slightly with temperature).
-
Electrode condition and calibration.
-
Ionic strength of the solution.
5. Applications in Pharmaceuticals
-
Ensuring product stability (pH affects drug solubility and degradation).
-
Controlling fermentation and biochemical processes.
-
Maintaining water quality in manufacturing.

