Importance of Data Integrity for Pharmaceutical Regulatory Agencies
Importance of Data Integrity for Pharmaceutical Regulatory Agencies
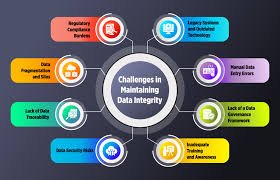
1. What is Data Integrity?
Data integrity means maintaining the accuracy, completeness, consistency, and reliability of data throughout its lifecycle, ensuring it is attributable, legible, contemporaneous, original, and accurate (ALCOA+ principles).
In pharmaceuticals, data integrity applies to all GMP-related data — manufacturing, testing, storage, distribution, and quality control.
2. Why Regulatory Agencies Focus on Data Integrity
-
Patient Safety & Product Quality
-
Regulatory agencies (FDA, EMA, MHRA, WHO) require evidence-based assurance that medicines are safe, effective, and of consistent quality.
-
If data is falsified, missing, or unreliable, patients may receive substandard or unsafe medicines.
-
-
Trust in Regulatory Decisions
-
Agencies rely on data submitted in NDA/ANDA/MAA filings, batch records, and QC test results to make approval decisions.
-
Integrity issues compromise regulators’ ability to trust company submissions.
-
-
Legal & Compliance Obligations
-
Data integrity is mandated by 21 CFR Part 11, Part 210/211 (USFDA), EU GMP Annex 11, and MHRA GxP guidance.
-
Violations often lead to FDA Form 483s, Warning Letters, import alerts, or consent decrees.
-
-
Prevention of Fraud & Misconduct
-
Agencies emphasize controls to prevent backdating, selective reporting, duplicate entries, or manipulation of electronic records.
-
Strong data integrity ensures transparency and accountability.
-
-
Global Supply Chain Reliability
-
Many countries rely on FDA/EMA/WHO approvals.
-
Data integrity lapses in one facility can disrupt global drug supply chains.
-
3. Consequences of Poor Data Integrity
-
Regulatory enforcement (483s, Warning Letters, import alerts, product recalls).
-
Loss of market authorization.
-
Damage to company reputation and patient trust.
-
Legal action, fines, or criminal liability in severe cases.
4. How Agencies Expect Companies to Maintain Data Integrity
-
Establish and implement robust data governance systems.
-
Train employees on ALCOA+ principles.
-
Ensure proper access controls for electronic systems.
-
Maintain audit trails and metadata.
-
Conduct periodic data integrity audits.
-
Implement CAPA when lapses are identified.
✅ Summary
Data integrity is non-negotiable for regulatory agencies because it underpins patient safety, product quality, and trust in the pharmaceutical industry. Without reliable data, regulators cannot make informed decisions, and companies cannot demonstrate compliance with cGMP.
🎓 Discover one of the best Complete Pharmaceutical Quality Assurance Course available —click below to explore the course that’s shaping future in QA Course skills.

