Good Practices for Air Handling Unit (AHU)
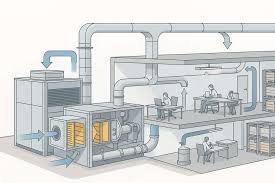
📌 Good Practices for Air Handling Unit (AHU) in Pharmaceuticals
1. Design & Installation
-
AHU must be designed as per GMP & cleanroom classification requirements.
-
Install HEPA/ULPA filters at terminal locations for sterile areas.
-
Provide air locks and pressure differentials to avoid cross-contamination.
-
Use smooth, cleanable surfaces to avoid microbial/particulate accumulation.
2. Operation & Monitoring
-
Maintain differential pressure (10–15 Pa) between areas of different grades.
-
Monitor and record temperature, RH, and differential pressure regularly.
-
Ensure uniform air distribution via proper ducting and diffuser design.
-
Validate and requalify AHUs at defined intervals.
3. Filter Management
-
Pre-filters, fine filters, and HEPA filters must be maintained and replaced as per schedule.
-
Perform integrity testing of HEPA filters (DOP/PAO test).
-
Ensure no bypass or leakages at filter joints.
4. Cleaning & Maintenance
-
Establish SOPs for periodic cleaning of AHU components (filters, coils, ducts, drain pans).
-
Use sanitized condensate drain pans to prevent microbial growth.
-
Calibrate and maintain instruments (differential pressure gauges, sensors).
-
Keep a preventive maintenance schedule for motors, fans, and belts.
5. Documentation & Compliance
-
Maintain logbooks for cleaning, filter changes, calibration, and maintenance.
-
Implement alarm systems for critical parameters (DP, temp, RH).
-
Ensure compliance with EU GMP Annex 1, US FDA, and WHO guidelines.

