Difference Between Paddle and Basket Dissolution
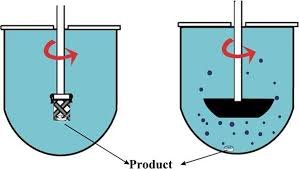
Here’s a clear comparison between Paddle and Basket dissolution methods used in pharmaceutical testing:
| Parameter | Paddle Method (USP Apparatus 2) | Basket Method (USP Apparatus 1) |
|---|---|---|
| Apparatus | Rotating paddle fixed at the center above the vessel bottom | Rotating cylindrical basket made of stainless-steel mesh |
| Dosage Form | Mainly for tablets, capsules (non-floating), and suspensions | Mainly for capsules, tablets that tend to float or disintegrate slowly |
| Rotation Speed | Commonly 50–75 rpm | Commonly 50–100 rpm |
| Sample Placement | Placed at the vessel bottom under the paddle | Placed inside the basket |
| Advantages | Simple setup, easier to clean, suitable for most solid dosage forms | Prevents floating of dosage form, ensures uniform exposure to medium |
| Disadvantages | Not suitable for floating dosage forms, risk of sticking to vessel bottom | More complex assembly, cleaning difficulty, possible clogging of mesh |
| Common Use in QC | Immediate-release and some modified-release dosage forms | Capsules or dosage forms prone to floating or sticking |
| USP Reference | USP <711> Dissolution | USP <711> Dissolution |
Key Points
-
Paddle Method is more commonly used in routine QC because it’s simple and versatile.
-
Basket Method is used when the product floats or has very slow disintegration.
-
Both methods must meet GMP and USP guidelines for validation and reproducibility.

