Data Integrity – A Major Problem in Pharmaceuticals
Data Integrity – A Major Problem in Pharmaceuticals
Data integrity has become one of the most critical issues in the pharmaceutical industry, directly impacting patient safety, product quality, and regulatory trust. Regulatory agencies like the FDA, EMA, and MHRA consistently cite data integrity violations as leading causes of FDA 483s, Warning Letters, import alerts, and product recalls.
Why Data Integrity Matters
-
Ensures that all data generated during drug development, manufacturing, testing, and distribution is accurate, complete, consistent, and reliable.
-
Protects patients by ensuring the safety, efficacy, and quality of medicines.
-
Provides regulators with confidence that products are manufactured under cGMP standards.
Common Data Integrity Issues in Pharma
-
Falsification or Fabrication of Data – Backdating, rewriting, or manipulating results.
-
Incomplete or Missing Records – Failure to document activities contemporaneously.
-
Inadequate Audit Trails – Computerized systems without proper tracking of changes.
-
Uncontrolled Paper Records – Use of loose sheets, unofficial logbooks, or “post-it” notes.
-
Selective Reporting – Recording only passing results (“testing into compliance”).
-
Poor Access Controls – Shared login IDs, lack of system security, or unauthorized data changes.
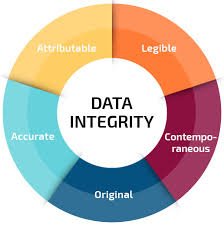
Regulatory Principles: ALCOA+
To ensure compliance, data should always be:
-
Attributable – Who performed the action and when.
-
Legible – Clear, permanent, and understandable.
-
Contemporaneous – Recorded at the time of activity.
-
Original – First capture of data.
-
Accurate – Error-free and truthful.
-
Complete, Consistent, Enduring, Available.
Impact of Data Integrity Failures
-
FDA Warning Letters and import alerts.
-
Production delays, drug shortages, and costly recalls.
-
Loss of reputation and market trust.
-
Legal penalties and, in severe cases, criminal liability.
How to Strengthen Data Integrity
-
Implement robust QMS with strict document control.
-
Use validated electronic systems with secure audit trails.
-
Conduct regular training on data integrity principles.
-
Perform internal audits and mock inspections to detect weaknesses.
-
Establish a culture of quality and transparency, encouraging employees to report issues without fear.
✅ Conclusion:
Data integrity is not just a regulatory requirement but a moral responsibility for pharmaceutical companies. A failure in this area compromises both patient safety and public trust. Ensuring strong data governance is the key to sustainable compliance and success.
🎓 Discover one of the best Complete Pharmaceutical Quality Assurance Course available —click below to explore the course that’s shaping future in QA Course skills.

