Q3: Impurities
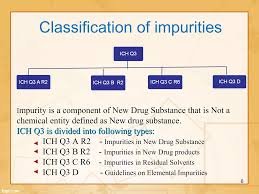
Elaboration:
Impurities are unwanted chemicals that remain with the active pharmaceutical ingredient (API) or develop during formulation and storage. According to ICH Q3A (for new drug substances) and ICH Q3B (for new drug products), impurities must be identified, quantified, and controlled to ensure product safety, efficacy, and quality.
Types of Impurities:
-
Organic Impurities – starting materials, intermediates, degradation products, by-products.
-
Inorganic Impurities – reagents, ligands, catalysts, heavy metals, filter aids, etc.
-
Residual Solvents – classified as Class 1 (to be avoided), Class 2 (to be limited), Class 3 (low toxic potential) as per ICH Q3C.
Control Strategy:
-
Establish impurity profile during development.
-
Set specification limits based on toxicity and qualification thresholds.
-
Regularly monitor via validated analytical methods (HPLC, GC, LC-MS).
-
Evaluate degradation pathways through stress studies (as per ICH Q1A).
Qualification of Impurities:
If impurities exceed the identification threshold, they must be qualified through:
-
Toxicological assessment, or
-
Reference to literature data, or
-
Safety studies.
Acceptance Criteria (Based on Maximum Daily Dose):
-
Identification Threshold
-
Reporting Threshold
-
Qualification Threshold
These thresholds differ depending on whether the daily dose is ≤2 g/day or >2 g/day.

