5 Steps of FDA Approvals
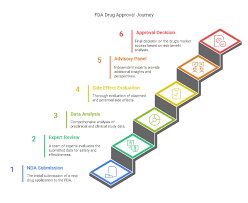
5 Steps of FDA Approvals
The U.S. Food and Drug Administration (FDA) follows a structured process to ensure that drugs are safe, effective, and high-quality before they reach patients.
1. Preclinical Testing (Laboratory & Animal Studies)
-
Conducted before human trials.
-
Purpose: Assess safety, toxicity, pharmacology, and efficacy signals.
-
Data generated is used to file an Investigational New Drug (IND) application with the FDA.
2. Investigational New Drug (IND) Application
-
Submitted to FDA before starting clinical trials.
-
Contains preclinical data, manufacturing details, and clinical trial protocols.
-
FDA reviews the IND (typically within 30 days) to ensure it is safe to proceed with human trials.
3. Clinical Trials (Human Testing)
Divided into 3 phases:
-
Phase I: Small group (20–100 healthy volunteers or patients). Focus on safety, dosage, and pharmacokinetics.
-
Phase II: Larger group (100–500 patients). Focus on efficacy and side effects.
-
Phase III: Large-scale studies (1,000+ patients). Confirm efficacy, monitor adverse effects, compare with standard treatments.
If successful, sponsor files a New Drug Application (NDA) or Biologics License Application (BLA).
4. FDA Review of NDA/BLA
-
FDA evaluates all submitted data:
-
Clinical trial results
-
Manufacturing and facility compliance (cGMP inspections)
-
Labeling (accuracy, clarity, safety info)
-
-
Expert advisory committees may be consulted.
-
Standard review: ~10 months; Priority review: ~6 months.
5. FDA Post-Market Surveillance (Phase IV)
-
After approval, FDA continues monitoring through:
-
MedWatch reports (adverse events)
-
Post-marketing studies (Phase IV commitments)
-
Inspections of manufacturing facilities
-
-
If risks outweigh benefits, FDA can issue label changes, restrictions, or product recalls.
✅ Summary
The 5 steps of FDA approvals are:
-
Preclinical Testing
-
IND Application
-
Clinical Trials (Phases I–III)
-
FDA Review of NDA/BLA
-
Post-Market Surveillance (Phase IV)
🎓 Discover one of the best Complete Pharmaceutical Quality Assurance Course available —click below to explore the course that’s shaping future in QA Course skills.

