Tablet Manufacturing Process: An Overview
🔹 Tablet Manufacturing Process: An Overview
Tablets are solid dosage forms containing one or more active pharmaceutical ingredients (APIs) with suitable excipients. The manufacturing process must ensure uniformity, stability, safety, and efficacy.
1. Pre-Formulation Studies
-
Study of API properties: solubility, stability, particle size, flow, compressibility.
-
Selection of excipients: diluents, binders, disintegrants, lubricants, glidants, coating materials.
-
Goal: Identify a suitable formulation strategy (direct compression, wet granulation, or dry granulation).
2. Formulation Approaches
There are three main manufacturing methods:
a) Direct Compression
-
Blend API + excipients → directly compressed into tablets.
-
Advantages: Simple, fast, cost-effective, fewer steps.
-
Limitations: Requires API with good flow and compressibility.
b) Wet Granulation
-
API + excipients mixed → binder solution added → wet mass formed → granulated → dried → sized → lubricated → compressed.
-
Advantages: Improves flow, compressibility, and content uniformity.
-
Limitations: Moisture/heat-sensitive drugs not suitable.
c) Dry Granulation (Slugging or Roller Compaction)
-
API + excipients compacted into slugs/compacts → milled → blended with lubricant → compressed.
-
Advantages: Suitable for moisture/heat-sensitive drugs.
-
Limitations: Higher cost, may have lower tablet strength than wet granulation.
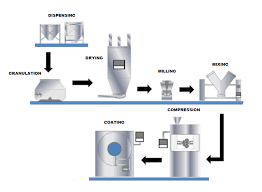
3. Unit Operations in Tablet Manufacturing
Step 1: Weighing and Dispensing
-
Accurate weighing of API and excipients in dispensing booth (to prevent cross-contamination).
Step 2: Blending / Mixing
-
Ensures uniform distribution of API with excipients.
-
Performed in V-blenders, double-cone mixers, or bin blenders.
Step 3: Granulation (if applicable)
-
Wet granulation: Uses fluid bed granulator or high-shear granulator.
-
Dry granulation: Uses roller compactor.
Step 4: Drying
-
Removes moisture from wet granules (fluid bed dryer, tray dryer).
-
Aim: Achieve optimal residual moisture for flow and compressibility.
Step 5: Milling / Sizing
-
Break down oversized granules, ensure uniform particle size.
Step 6: Final Blending / Lubrication
-
Add lubricants (e.g., magnesium stearate), glidants (colloidal silica), disintegrants.
Step 7: Compression
-
Granules or blends compressed into tablets using rotary or single-punch tablet press.
-
Parameters: hardness, thickness, weight, friability.
Step 8: Tablet Coating (optional)
-
Film coating: Thin polymeric layer for protection, taste masking, controlled release.
-
Sugar coating: Traditional, thicker, less common now.
-
Enteric coating: Protects drug from stomach acid.
Step 9: Packaging
-
Blister packs, bottles, or strips under controlled conditions.
-
Ensures stability, patient compliance, tamper resistance.
4. In-Process & Quality Control
-
Blend uniformity
-
Granule flow, bulk/tapped density
-
Tablet weight variation, hardness, friability, disintegration, dissolution
-
Content uniformity, assay, microbial limits
-
Visual inspection for defects (chipping, capping, picking, lamination).
5. Key Considerations
-
Compliance with GMP, ICH, and pharmacopeial standards (USP, EP, IP).
-
Critical Process Parameters (CPPs) must be controlled to achieve desired Critical Quality Attributes (CQAs).
-
Process Analytical Technology (PAT) and Quality by Design (QbD) approaches increasingly applied.
✅ Summary
The tablet manufacturing process involves:
-
Pre-formulation & formulation development
-
Blending, granulation, drying, sizing, and lubrication (depending on method)
-
Compression and coating
-
Packaging & QC testing
The chosen method (direct compression, wet granulation, dry granulation) depends on the API properties and product requirements.
🎓 Discover one of the best Complete Pharmaceutical Production Course available —click below to explore the course that’s shaping future Production Course skills.
https://trcjw.on-app.in/app/oc/338669/trcjw

