Risk Assessment for the Purified Water System in Pharmaceuticals
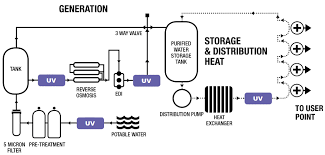
Risk Assessment for Purified Water System in Pharmaceuticals
1. Objective
To identify, evaluate, and control risks associated with the design, operation, and maintenance of the Purified Water System (PWS), ensuring compliance with GMP, pharmacopeial standards, and data integrity.
2. Risk Assessment Methodology
-
Follow ICH Q9 (Quality Risk Management).
-
Tools: FMEA (Failure Mode and Effects Analysis), HACCP, Risk Ranking & Filtering.
-
Risk = Probability × Severity × Detectability.
3. Risk Areas & Examples
A. Design Stage Risks
-
Material of construction: Non-compliant SS grade (should be SS 316L with sanitary finish).
-
Dead legs > 1.5D (cause microbial growth).
-
Improper slopes (should be ≥1:100 for drainability).
-
Inadequate vent filtration on storage tanks.
Risk Impact: Biofilm formation, endotoxin contamination, system failure.
B. Operation & Performance Risks
-
Temperature control: If loop temp < 70 °C (hot) or stagnant in ambient system → microbial proliferation.
-
Sanitization failure: Ineffective hot water/chemical/ozone cycles.
-
Flow velocity: < 1.0 m/s may cause biofilm formation.
-
Inadequate monitoring: No online conductivity/TOC sensors.
Risk Impact: Microbial contamination, OOS results, batch rejection.
C. Maintenance & Monitoring Risks
-
Filter integrity not checked (tank vent, pre-filters).
-
Calibration lapses of conductivity, TOC, and flow sensors.
-
Improper sampling techniques → false positives/negatives.
-
Delayed media replacement in pre-treatment (sand, carbon, softener).
Risk Impact: Non-compliance, regulatory observations.
4. Risk Mitigation / Control Measures
-
Design Controls
-
Sanitary SS 316L piping with orbital welding.
-
Dead-leg free design, loop slope ≥1:100.
-
Hygienic tank design with spray ball.
-
-
Operational Controls
-
Continuous recirculation at ≥ 1.0 m/s velocity.
-
Maintain hot loop ≥70–80 °C or validated sanitization frequency.
-
Use validated on-line monitoring (Conductivity, TOC, Temp, Flow).
-
-
Maintenance & Monitoring
-
Preventive maintenance schedule for filters, pumps, valves.
-
Periodic calibration of sensors.
-
Routine microbiological and chemical testing at user points.
-
Trend data review for early detection of drift.
-
-
Procedural Controls
-
SOPs for sanitization, sampling, and monitoring.
-
Qualified personnel with periodic training.
-
Deviation/CAPA management system.
-
5. Risk Assessment Outcome
-
Risks are reduced to Acceptable Level through:
-
Design qualification (DQ),
-
Installation qualification (IQ),
-
Operational qualification (OQ),
-
Performance qualification (PQ), and
-
Ongoing monitoring (Continual Validation).
-
6. Documentation
-
Risk Assessment Report (RA).
-
Validation Master Plan (VMP).
-
Change control & deviation records.
-
Trending reports of water quality.
-
Audit trail & QA approval.
✅ In short:
Risk assessment of a purified water system focuses on design (material, dead legs, slope), operation (temperature, velocity, sanitization), and monitoring (calibration, sampling, trending). Controls include good design, validated monitoring, preventive maintenance, and strong QMS practices.
🎓 Discover one of the best Pharmaceutical Microbiology course available —click below to explore the course that’s shaping future Microbiology course skills.

