Principle and Working of Gas Chromatography
1. Principle of Gas Chromatography
Gas Chromatography is based on the principle of partitioning of components between a mobile phase (inert carrier gas) and a stationary phase (solid or liquid supported on a solid).
-
Mobile phase: An inert gas (e.g., helium, nitrogen, hydrogen) that carries the sample through the column.
-
Stationary phase: Coated inside the column (capillary or packed) — either a solid adsorbent or a liquid film on an inert support.
Separation Principle:
Each compound in the injected sample interacts differently with the stationary phase and travels at different speeds through the column.
-
Components with less interaction with the stationary phase move faster and elute earlier.
-
Components with more interaction move slower and elute later.
The time taken for a component to travel through the column and reach the detector is called the retention time (tR) — characteristic for each compound under fixed conditions.
2. Working of Gas Chromatography
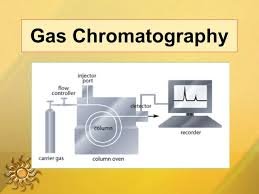
The GC process occurs in six main steps:
-
Sample Injection
-
The sample (liquid or gaseous) is injected into the injector port using a microsyringe.
-
The injector is kept at a high temperature to vaporize the sample instantly.
-
-
Vaporization & Mixing with Carrier Gas
-
The vaporized sample mixes with the carrier gas (mobile phase) and enters the column.
-
-
Separation in the Column
-
The column (in an oven) contains the stationary phase.
-
Oven temperature is controlled — either isothermal or temperature programmed — to optimize separation.
-
Components partition between the mobile gas phase and stationary phase, separating based on volatility and polarity.
-
-
Detection
-
As separated components exit the column, they pass through a detector (e.g., Flame Ionization Detector [FID], Thermal Conductivity Detector [TCD], Electron Capture Detector [ECD], etc.).
-
The detector generates a signal proportional to the concentration of each component.
-
-
Signal Processing
-
The signal is sent to a computer/data system, which records it as peaks on a chromatogram.
-
The position (retention time) identifies the compound, and peak area/height quantifies it.
-
-
Data Interpretation
-
The chromatogram is analyzed for qualitative (identification) and quantitative (amount) purposes.
-
Schematic Flow of GC:
Sample Injection → Vaporization → Carrier Gas Transport → Column Separation → Detection → Data Output
3. Applications
-
Pharmaceuticals: Residual solvent analysis, purity testing
-
Environmental: Air pollutants, pesticide residues
-
Food: Flavor and fragrance profiling
-
Forensic: Alcohol and drug analysis in biological samples
-
Petrochemical: Hydrocarbon analysis
✅ Key Takeaway:
Gas Chromatography separates compounds based on differences in volatility and interactions with the stationary phase, using an inert gas to transport the sample through a temperature-controlled column, with detection at the end.

