Recovery Factor in Cleaning Validation: Procedure and Importance
Recovery Factor in Cleaning Validation: Procedure and Importance
In the cleaning validation, the recovery factor plays a crucial role in determining how effectively residues can be removed from equipment surfaces. This metric ensures the precision of analytical methods and confirms that cleaning procedures are thoroughly validated. In this article, we are going to see the importance of the recovery factor and the procedure involved.
– Accuracy: It verifies that analytical methods can accurately detect and quantify residues at specified levels.
– Effectiveness: Confirms that the cleaning process is effective in removing the residues
– Compliance: It must ensure the cleanliness of the equipment meets the regulatory standards and safety requirements
Step-by-Step Procedure for determining the recovery factor
1. Testing surface selection
– Identify all surfaces in contact with the product. Common materials include stainless steel, glass, and plastic.
2. Preparation of Test Solutions
– Create standard solutions containing known concentrations of the residue, such as active pharmaceutical ingredients or excipients.
3. Application of Test Solutions
– Apply a precise amount of the test solution to the selected surfaces using methods like pipetting or spraying.
4. Drying
– Allow the test solution to dry on the surfaces, simulating the conditions encountered during actual manufacturing processes.
5. Sampling
– Collect residue samples from the test surfaces using appropriate methods:
– Swabbing: Systematically wipe the surface with pre-moistened swabs.
– Rinsing: Rinse the surface with a specific volume of solvent and collect the rinse solution.
6. Analysis
– Analyze the collected samples using validated analytical techniques such as High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), or Total Organic Carbon (TOC) analysis.
7. Calculation of Recovery Factor
– Measure the amount of residue recovered from the surface.
– Calculate the recovery factor using the formula:
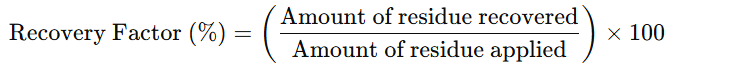
8. Documentation
– Thoroughly document all procedures, calculations, and results in a validation report, detailing the test surface material, residue concentration, sampling method, and analytical techniques employed.
9. Evaluation and Acceptance Criteria
– Compare the recovery factor against predefined acceptance criteria. Typically, recovery rates between 70% and 120% are considered acceptable, although this range may vary based on specific regulatory guidelines.
By adhering to the above procedure, pharmaceutical companies can ensure their cleaning validation processes are robust and reliable, thereby guaranteeing that their equipment is free of contaminants and residues.

