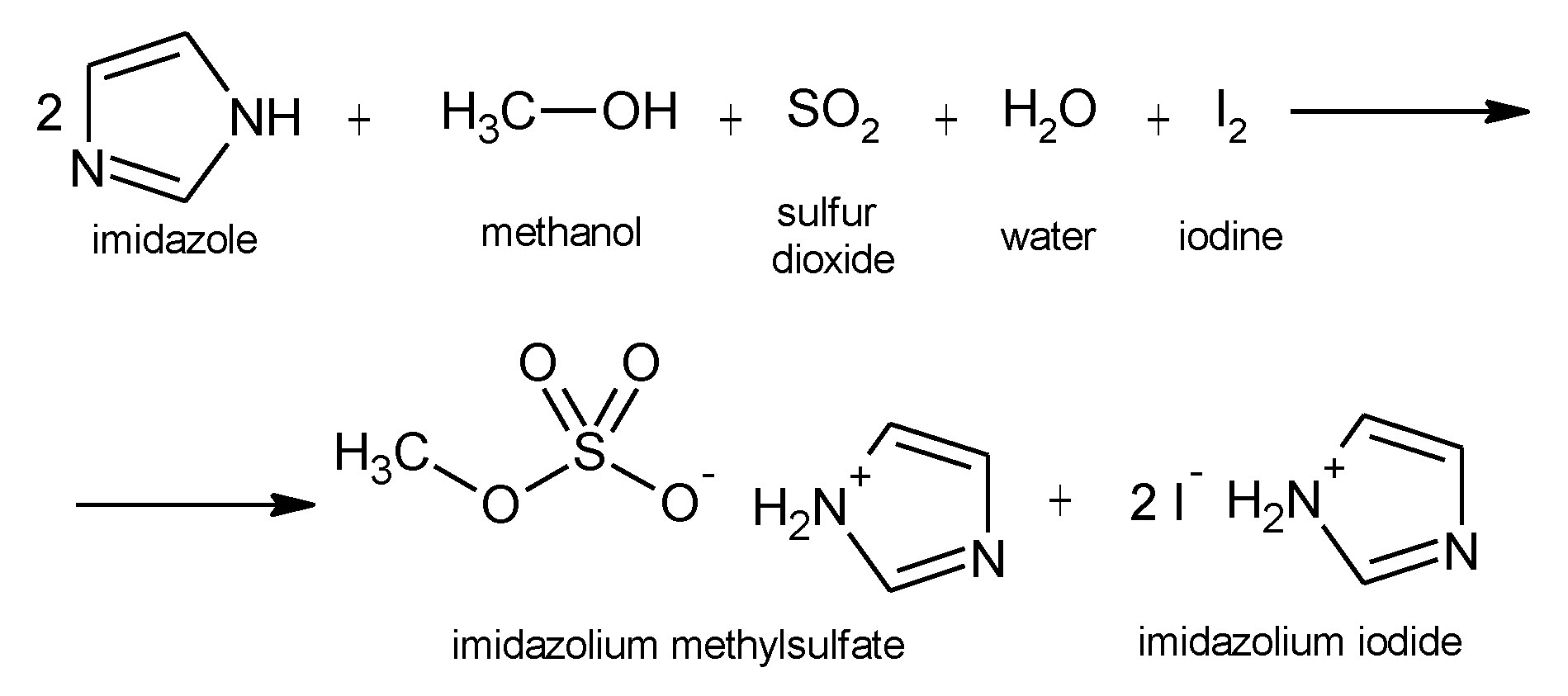
what is Karl Fischer principle and reaction ,describe it?
The fundamental principle behind it is based on the Bunsen reaction between iodine and sulfur: dioxide in an aqueous medium. Water and iodine are consumed in a 1:1 ratio in the above reaction. Once all of the water present is consumed, the presence of excess iodine is detected volta metrically by the titrator’s indicator electrode. That signals the end-point, of the titration.
The amount of water present in the sample is calculated based on the concentration of iodine in the Karl Fisher titrating reagent (i.e., titer) and the amount of Karl Fisher reagent consumed in the titration.